Introduction: Urgent Recall of Boots Paracetamol due to Packaging Error
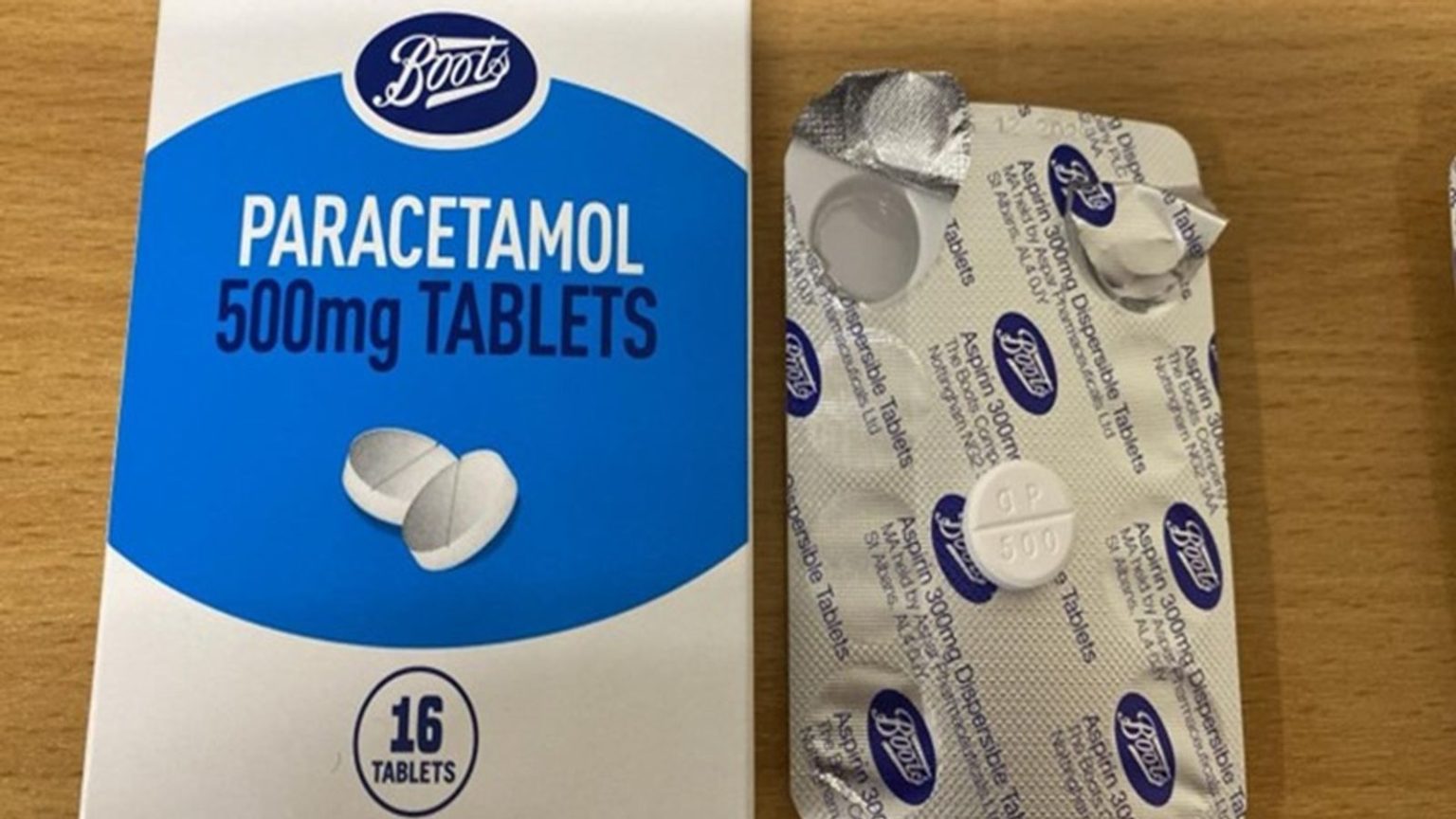
In a concerning turn of events, Boots has issued an urgent recall of certain paracetamol packets due to a critical packaging error. This error has led to confusion, as packets labeled as "Aspirin 300mg Dispersible Tablets" actually contain 500mg paracetamol tablets. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has stepped in, advising consumers to take immediate action to ensure their safety.
Section 1: Details of the Packaging Error
The affected packets, part of Boots’ 16-tablet paracetamol boxes, bear the incorrect label of aspirin. The specific batch number to watch out for is 241005, located on the bottom of the box. This batch number is crucial for identification, as it directly indicates the incorrect packaging. Customers are urged to check their purchases carefully to avoid any potential medication mix-ups.
Section 2: Recall Notice and Necessary Actions
Boots and the MHRA haveissued a clear recall notice, instructing customers with the affected batch to cease use immediately. The steps to follow are straightforward: stop using the product, return it to a Boots store for a full refund, and if unsure or if side effects are experienced, consult a healthcare professional. Dr. Stephanie Millican, MHRA’s Deputy Director, emphasizes the importance of compliance, stating that immediate action is vital to prevent incorrect dosages.
Section 3: The Importance of Compliance
Compliance with this recall is not just a precaution; it is essential to prevent potential health risks. Mixing up paracetamol and aspirin can lead to serious health issues, especially for those with certain medical conditions or those taking other medications. The MHRA’s Deputy Director underscores that checking medication packaging is a critical step in ensuring personal health and safety.
Section 4: Customer Reactions and Communication
Boots utilized their official Facebook page to inform customers about the recall, demonstrating a proactive approach to communication. The supplier, Aspar Pharmaceuticals Limited, has confirmed that the tablets are indeed paracetamol and not aspirin, and is conducting a thorough investigation. This transparency aims to reassure customers and maintain trust.
Section 5: Wider Implications and Quality Control
This incident raises broader questions about quality control in medication packaging. Trust in pharmaceutical products is paramount, and such errors can erode this trust. The recall serves as a reminder of the need for rigorous quality checks and the importance of a prompt response when issues arise.
Conclusion: Key Takeaways and Final Advice
In summary, the recall of Boots’ paracetamol packets due to a packaging error is a serious issue requiring immediate attention. Consumers are advised to check for batch number 241005, cease use if affected, and seek refunds. The situation highlights the critical nature of accurate labeling and the importance of consumer vigilance. Always verify your medication’s details, and if in doubt, consult a healthcare professional. Stay informed, and prioritize your health and safety above all else.