A New Era in Pain Management: The FDA Approval of Journavx
1. Breaking the Cycle: A Non-Opioid Solution for Acute Pain
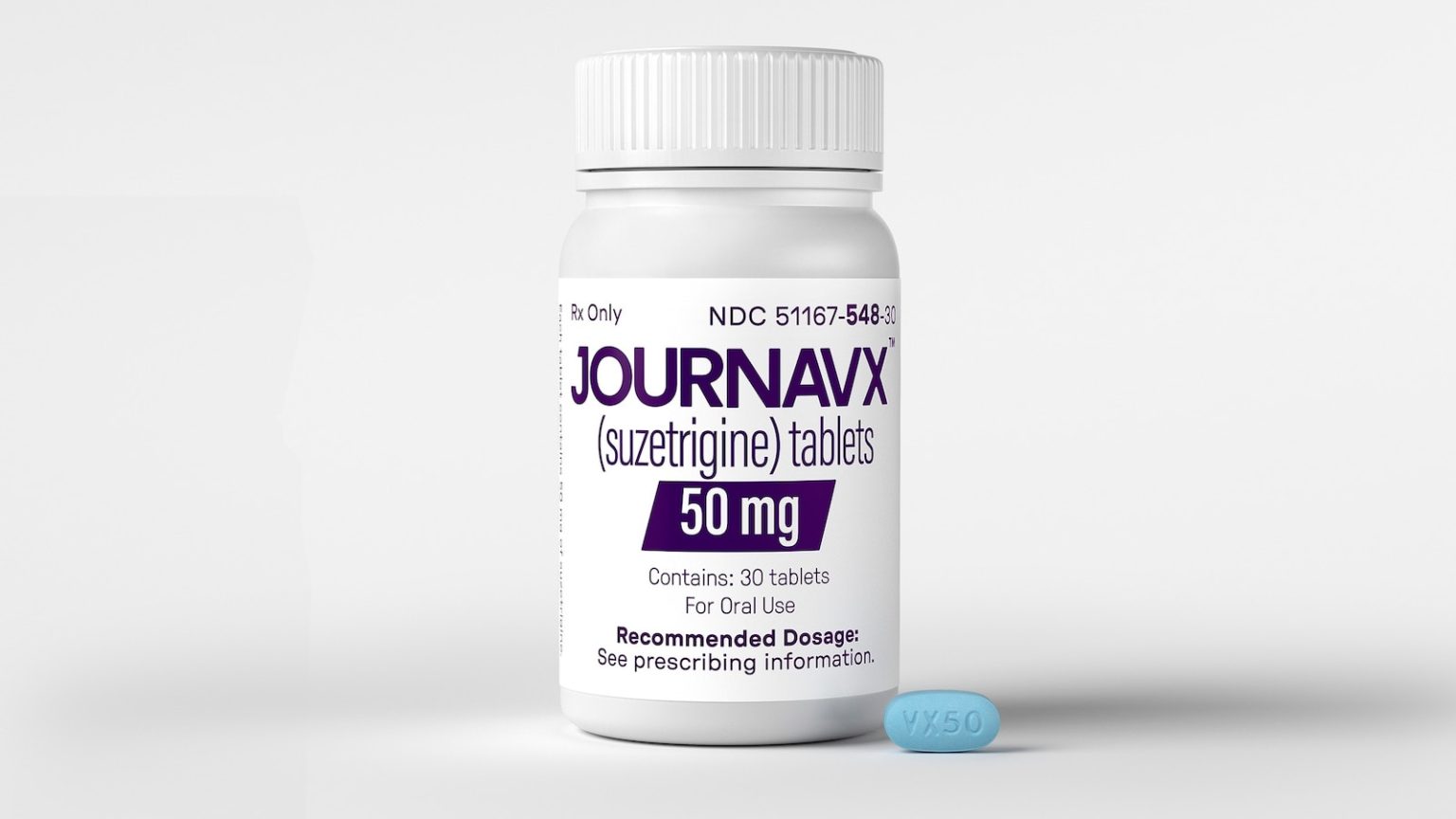
The U.S. Food and Drug Administration (FDA) has marked a groundbreaking moment in the history of pain management with the approval of Journavx (suzetrigine), a revolutionary new prescription pain medication designed for adults suffering from moderate to severe acute pain. Developed by Vertex Pharmaceuticals, Journavx stands out as a non-opioid alternative, offering hope to millions of people who have long relied on addictive opioid-based treatments. This approval is a significant public health milestone, as it introduces the first non-opioid drug class for acute pain in over two decades. The FDA’s decision comes at a time when the nation is grappling with the opioid crisis, making this breakthrough particularly timely and impactful.
Dr. Jacqueline Corrigan-Curay, the acting director of the FDA’s Center for Drug Evaluation and Research, emphasized the importance of this approval: “Today’s decision provides patients with a new treatment option and helps mitigate the risks associated with opioid use.” Journavx not only offers a safer alternative but also promises to reduce the nation’s reliance on opioids, which have been a leading cause of addiction and overdose deaths.
2. Innovation in Action: How Journavx Works
Journavx works by targeting the NaV1.8 pain signal in the peripheral nervous system, a mechanism that sets it apart from traditional opioid painkillers. Unlike opioids, which act on the central nervous system and carry a high risk of addiction, Journavx specifically inhibits pain signals at their source—without affecting the brain or central nervous system. This unique approach ensures that the drug is non-addictive while still providing effective pain relief.
The results of two clinical trials highlight Journavx’s efficacy. In these studies, the drug reduced moderate to severe acute pain by about 50% within 48 hours, with patients experiencing meaningful relief in as little as two to four hours—compared to eight hours for the placebo group. These findings demonstrate that Journavx is not only effective but also fast-acting, making it an ideal option for acute pain scenarios.
Moreover, Journavx was found to be as effective as hydrocodone, a commonly prescribed opioid, in reducing acute pain. This equivalence in efficacy, combined with its non-addictive properties, positions Journavx as a game-changer in the field of pain management.
3. A Safer Alternative: Clinical Trials and Patient Feedback
The clinical trials for Journavx involved adults aged 18 to 80, testing the drug’s effectiveness across a range of acute pain conditions, both surgical and non-surgical. The results were overwhelmingly positive, with over 80% of participants rating Journavx as “good,” “very good,” or “excellent” for pain relief. This high level of patient satisfaction underscores the drug’s potential to improve the quality of life for those struggling with acute pain.
One of the most promising aspects of Journavx is its safety profile. Unlike opioids, which can lead to respiratory depression, dependence, and overdose, Journavx does not carry these risks. This makes it a much safer option for patients, particularly those who are at risk of addiction or those who require long-term pain management.
However, it’s important to note that Journavx may not be suitable for everyone. The drug cannot be taken with certain medications that strongly inhibit a specific liver enzyme, and patients are advised to avoid grapefruit while on Journavx. As with any new medication, healthcare providers will need to evaluate individual patients to determine if Journavx is the right choice for them.
4. Expert Insights: A Safer Future for Pain Management
The approval of Journavx has been welcomed by medical experts, who see it as a critical step forward in addressing the opioid crisis. Dr. Jianguo Cheng, a professor of anesthesiology at the Cleveland Clinic, highlighted the drug’s potential to transform acute pain management. “Journavx offers a safer option for managing moderate-to-severe acute pain,” he said. “It provides rapid relief and can be integrated into postoperative pain protocols or acute pain scenarios where immediate relief is critical.”
Dr. Cheng also noted that by effectively managing acute pain, Journavx may help prevent the transition to chronic pain, reducing the need for long-term pain management strategies. This is a key benefit, as chronic pain is often more challenging to treat and can significantly impact a patient’s quality of life.
The introduction of Journavx also opens up new possibilities for postoperative care. Surgeons and anesthesiologists are eager to explore how this drug can be incorporated into pain management plans, particularly for patients who are at higher risk of opioid addiction.
5. The Bigger Picture: The Impact on Public Health
The approval of Journavx is more than just a medical breakthrough—it’s a powerful tool in the fight against the opioid epidemic. Opioids have claimed countless lives over the years, and the need for non-addictive alternatives has never been more urgent. Journavx represents a step in the right direction, offering a safer way to manage acute pain without the risks associated with opioids.
By reducing reliance on opioids, Journavx could help prevent new cases of addiction and overdose. This, in turn, could have a ripple effect throughout communities, leading to fewer lives lost and less strain on healthcare systems.
Moreover, the success of Journavx could pave the way for further innovation in pain management. As researchers continue to explore new non-opioid treatments, the hope is that even more effective and safer options will become available in the future.
6. Conclusion: A Brighter Future for Pain Relief
The FDA’s approval of Journavx marks the beginning of a new chapter in pain management, one that prioritizes safety and efficacy. This drug not only addresses the urgent need for non-opioid alternatives but also offers a glimmer of hope for those who have long suffered from acute pain.
As Journavx becomes available to patients, it will be crucial to monitor its real-world performance and ensure that it is accessible to those who need it most. Healthcare providers, policymakers, and patients must work together to make sure that this groundbreaking medication is used responsibly and effectively.
In the end, the approval of Journavx is more than just a medical advancement—it’s a testament to the power of innovation and the unwavering commitment to improving human health. As we look to the future, it’s clear that the landscape of pain management is changing for the better, and Journavx is leading the way.