Moderna Secures $590 Million Federal Grant to Accelerate mRNA-Based Bird Flu Vaccine Development
In a significant step toward safeguarding public health, Moderna has been awarded approximately $590 million by the U.S. Department of Health and Human Services (HHS) to fast-track the development of an mRNA-based bird flu vaccine. This funding is part of a broader effort to enhance the nation’s preparedness against potential influenza pandemics, including the H5N1 avian influenza virus. Health officials emphasized that this investment will not only accelerate the creation of vaccines for currently circulating strains but also expand research into other influenza subtypes that could pose future threats.
The announcement comes as the U.S. faces a growing number of bird flu cases in humans, with 67 confirmed cases reported as of Friday, including the first recorded death in Louisiana earlier this month. While the risk of human-to-human transmission remains low, the unpredictable nature of avian flu variants has prompted the Biden-Harris Administration to prioritize vaccine development. HHS Secretary Xavier Becerra highlighted the importance of staying ahead of potential outbreaks, ensuring Americans have the tools needed to remain safe.
The Science Behind mRNA Technology: A Game-Changer for Vaccine Development
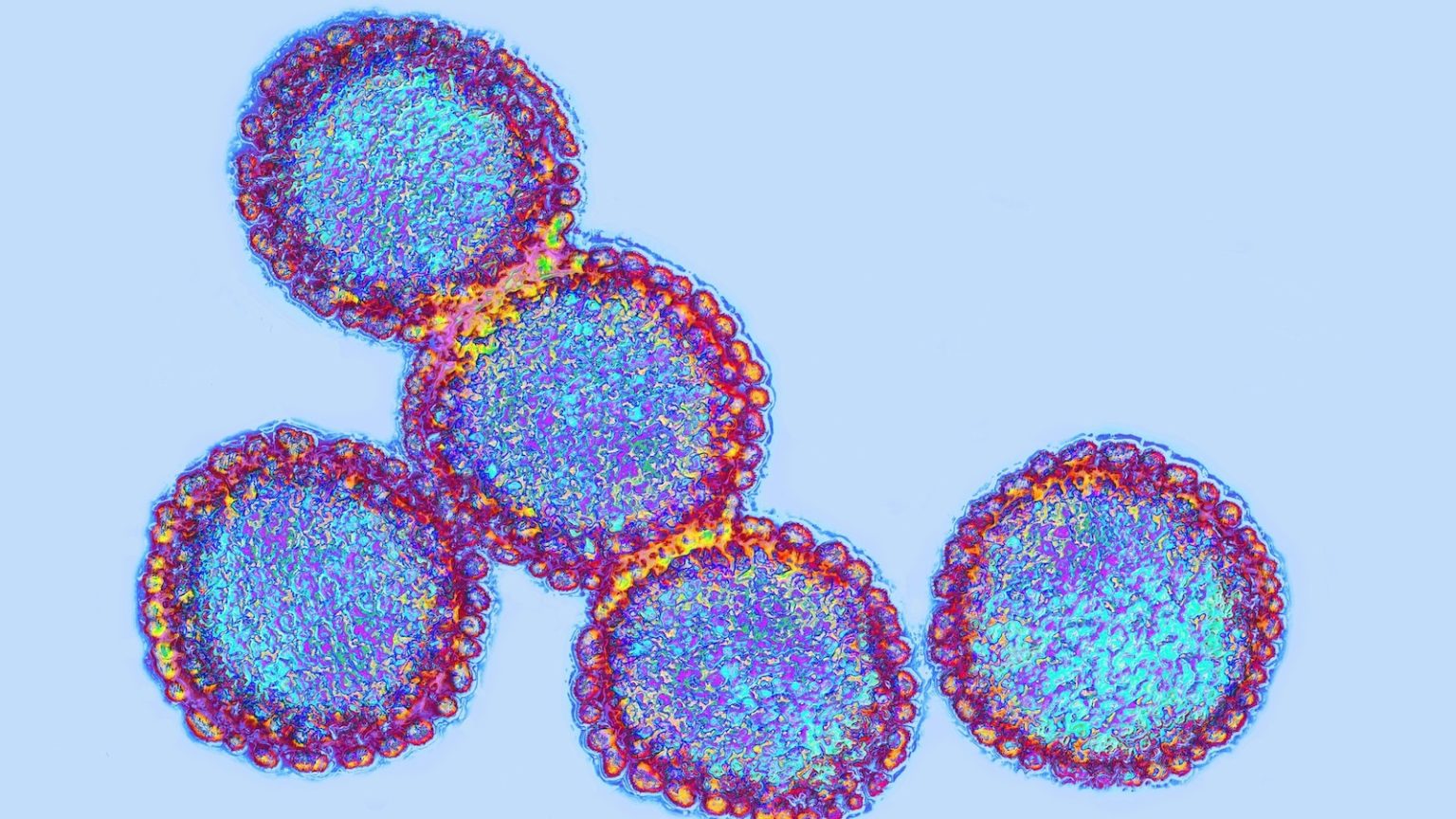
Central to Moderna’s efforts is the use of mRNA (messenger RNA) technology, the same innovative approach that played a pivotal role in the rapid development of COVID-19 vaccines. Unlike traditional vaccines that rely on weakened or inactivated viruses, mRNA vaccines work by teaching the body to produce specific proteins that trigger an immune response. This method allows scientists to design and manufacture vaccines at an unprecedented speed, a critical advantage when responding to emerging health threats.
The HHS grant will enable Moderna to expand clinical studies for up to five additional subtypes of pandemic influenza, further bolstering the nation’s defenses against a wide range of flu strains. This multi-pronged approach reflects the federal government’s commitment to preparing for not just the current bird flu outbreak but also future pandemics. By leveraging cutting-edge technology, Moderna and its partners aim to create vaccines that are both effective and adaptable to new challenges.
The Federal Government’s Proactive Approach to Bird Flu Preparedness
The U.S. government has already taken significant steps to combat the bird flu threat. In addition to the $590 million awarded to Moderna, the company previously received $176 million in July 2024 to expedite the development of an mRNA-based bird flu vaccine. These investments complement the existing stockpile of traditional bird flu vaccines, which are already being stockpiled as a precautionary measure. Officials anticipate having 10 million ready-to-use doses available by early 2025, ensuring a robust response should the situation escalate.
The Current Bird Flu Outbreak: Understanding the Risks and Responses
Since April 2024, bird flu cases among humans have been reported across the U.S., with the majority of infections linked to contact with infected poultry or cattle. The CDC has confirmed 67 cases as of Friday, and while most patients have experienced mild symptoms, the recent death in Louisiana serves as a stark reminder of the potential dangers of the virus. Health officials have repeatedly stressed that the risk of human-to-human transmission remains low, but the unpredictable nature of avian flu variants necessitates vigilance and proactive measures.
Public health agencies, including the CDC, are closely monitoring the situation and urging individuals who work with or come into contact with birds to take precautions. The federal government’s dual strategy of investing in next-generation mRNA vaccines while stockpiling traditional vaccines underscores its commitment to safeguarding the nation’s health and staying ahead of emerging threats. By combining innovation with preparation, the U.S. aims to minimize the impact of bird flu and other influenza variants in the years to come.
Looking Ahead: The Importance of Preparedness and Innovation
The federal government’s decision to invest heavily in Moderna’s mRNA-based bird flu vaccine reflects a broader recognition of the critical role innovation plays in protecting public health. By accelerating the development of vaccines that can adapt to new and emerging strains, the U.S. is positioning itself to respond more effectively to future pandemics. This effort not only addresses the immediate threat of bird flu but also lays the groundwork for tackling other infectious diseases that may arise.
As the nation continues to navigate the challenges posed by bird flu and other influenza viruses, collaboration between government agencies, pharmaceutical companies, and healthcare providers will be essential. By combining cutting-edge technology, scientific expertise, and a proactive approach to preparedness, the U.S. is taking significant strides toward ensuring the safety and well-being of its citizens.