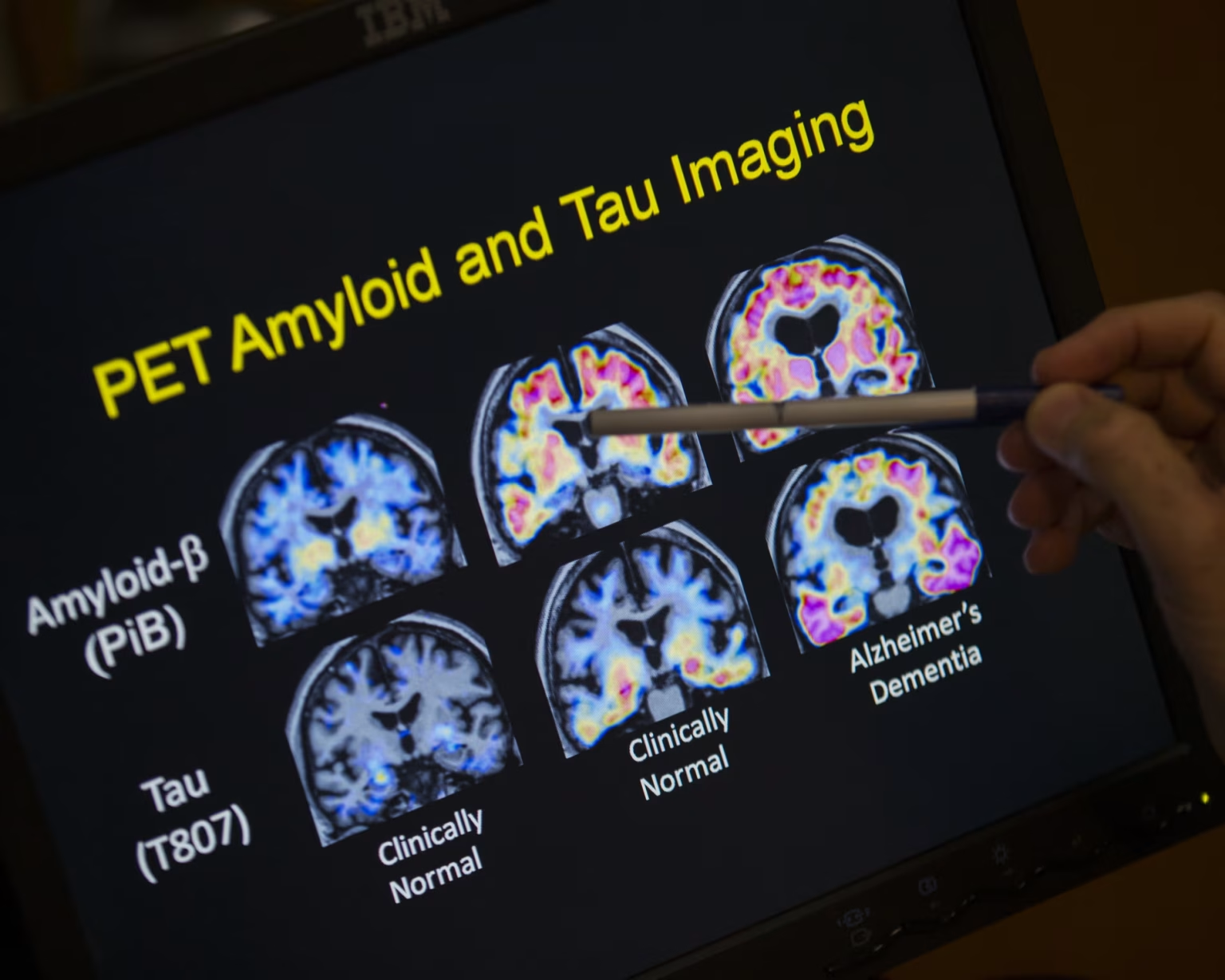
Australia’s Therapeutic Goods Administration has approved a new drug called donanemab, marketed as Kisunla, to treat early-stage Alzheimer’s disease. Developed by Eli Lilly, this drug targets amyloid proteins in the brain, which are believed to contribute to Alzheimer’s progression. This approval marks the first new Alzheimer’s treatment in 25 years and the first that slows the disease rather than just easing symptoms.
Donanemab is administered through an intravenous infusion every four weeks for up to 18 months. Clinical studies have shown it can slow the rate of cognitive decline by about one-third in patients with early symptomatic Alzheimer’s disease. However, only about 10 to 20 percent of people with dementia will be eligible for this treatment because it requires a specific genetic profile and early diagnosis.
Patients must be in the mild cognitive impairment stage to qualify. Those with two copies of the ApoE ε4 gene, which increases the risk of brain swelling and bleeding, are excluded. Before treatment, patients need genetic testing and MRI scans to ensure there are no signs of brain bleeding or swelling. Continuous monitoring with MRI is also required during therapy.
Diagnosis must be confirmed by lumbar puncture or amyloid PET scans, which are not covered by Medicare. The drug is not yet listed on the Pharmaceutical Benefits Scheme, so patients will currently have to pay out of pocket. The total cost of treatment, including the drug, specialist fees, infusions, and scans, could exceed $80,000.
Eli Lilly has applied for the drug to be added to the Pharmaceutical Benefits Scheme, with a review expected in July. The federal government is still considering reimbursement options and the logistics of required patient monitoring.
Some experts debate whether the benefit of slowing decline by a third justifies the risks and high costs. Patients and their families will need to weigh these factors carefully when deciding on treatment. Dementia remains a leading cause of death in Australia, making advances in treatment important for many.
The Australian Dementia Network is running trials for blood tests that can help general practitioners diagnose Alzheimer’s earlier. This could improve access to treatment by increasing early diagnosis capacity.